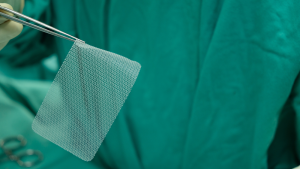
By David B. Rheingold, Esq. The well-regarded Judge Edmund A. Sargus, Jr., ordered the parties on February 29, 2024, to a mediation process scheduled for approximately three
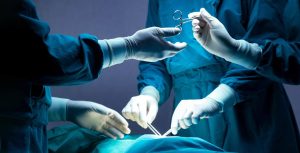
By David B. Rheingold On February 29, 2024, the judge overseeing the federal Bard hernia mesh litigation ordered the parties to mediation with a court-appointed special negotiating
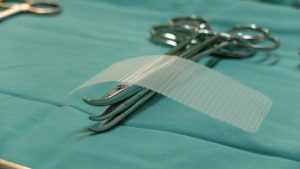
By David B. Rheingold, Esq. Hernia mesh settlements have recently happened for various products. Will a new Bard settlement happen soon? Federally ordered mediation on March 25

Exactech is facing legal challenges with over 1,100 lawsuits in federal courts and 400 in state courts nationwide.[i] These lawsuits allege manufacturing defects in knee,
The Paragard Multidistrict Litigation is moving forward after US District Court Judge issued the latest scheduling order concerning deadlines for discovery, including disclosure of experts
Of the more than 1,100 Exactech knee replacement lawsuits filed across the country, twelve will be selected for bellwether trials beginning as early as June

By Benjamin Pace In a significant development, two members of the Senate Judiciary Committee, Senators Dick Durbin (D-Ill.) and Richard Blumenthal (D-Conn.), are urging a
Megadyne has recently received reports of 63 injuries (and counting) related to their MEGA 2000 and MEGA SOFT Reusable Patient Electrode devices.[1] The list of
The Actions against Bard seek to hold the device manufacturer liable for injuries caused by their wrongful conduct in connection with the design, development, manufacture,
Also commonly referred to as Injection Parts or Port-a-Catheters, the Bard PowerPort products at issue in these lawsuits are implantable vascular access devices designed to
Once you contact Rheingold Giuffra Ruffo Plotkin & Hellman LLP for guidance, you will be treated with the utmost professionalism, commitment, and respect. Our first
In 2014, Covidien was acquired by Medtronic and now exists as one of Medtronic’s subsidiaries. Covidien hernia mesh products have been around for about two
There are dozens of models in the Covidien Symbotex line of hernia mesh implants that come in a variety of shapes and sizes. With so
The value of a Covidien hernia mesh lawsuit varies on a case-to-case basis and is determined by weighing a number of factors. These factors include,
On May 30, 2023, US Magistrate Judge Marcia M. Henry ordered that the discovery process be expedited in the Exactech mass tort litigation so that
Exactech is a multinational medical device manufacturer that designs and manufactures orthopedic medical devices such as joint implants. Recently in 2022, several thousand Exactech hip,
Q. What is a mass tort lawsuit? A. A mass tort lawsuit involves a product that causes injury to many people. Also known as a
The value of an Exactech recall lawsuit varies on a case-to-case basis and is determined by weighing a number of factors. Determinative factors include, but
By David B. Rheingold, Esq. STRATTICE™ biologic mesh is made with pig tissue as opposed to synthetic material. Litigations are located in New Jersey State
Hernia repair is a very common surgery with over one million operations performed annually in the United States.[i][ii] Surgeons have several options when it comes
Paragard, a type of intrauterine device (IUD) manufactured by Teva Pharmaceuticals, has been the subject of numerous lawsuits in recent years. These lawsuits have claimed that the
Last week the annual shareholder meeting of Johnson & Johnson took place at its headquarters in New Brunswick, New Jersey. Despite the proclamation of approvals
By Maria Markou, Esq. Honorable Judge Brian Martinotti supervises in a multi-county litigation (MCL) in Bergen County, where more than half of the nearly 1,500
By Paul D. Rheingold A respected securities analyst, Northland Securities, Inc. released a March 2013 advisory regarding Intuitive Surgical’s stock valuation. Due to the shortcomings
Many patients and physicians in New York are taking note of claims of injuries caused by robotic medical devices that are not being reported to
Reuters (5/29, Dye) reports that Boston Scientific was ordered by a Delaware court on Thursday to pay $100 million to a woman who claimed injuries
A class I recall was issued in April, 2015, for all HeartWare Ventricular Assist Systems (VAS) currently in use. The HeartWare VAS helps deliver blood
By: Kelda Doherty It is no surprise to our firm that Johnson & Johnson, the largest manufacturer of health care products in the world, has
A California appeals court upheld an award of $3.61 million against C.R. Bard in a transvaginal mesh case. The Plaintiff received the Avaulta Plus transvaginal
A federal jury in West Virginia awarded four plaintiffs $14.5 million in compensatory damages and $4 million in punitive damages in a Boston Scientific TVM
Johnson & Johnson, the manufacturer of drugs and medical devices among other products, was ordered by a Jury in California to pay $5.7 million. The
A recent Wall Street Journal report found that 14 deaths were tied to Medtronic’s SynchroMed drug-infusion pumps. The Department of Justice (DOJ), in conjunction with
FDA announced a Class I Recall of the Tiger Paw System II, produced by Maquet Medical Systems. The Class I is the most serious FDA